Kinesin Stepping Mechanism
Faculty MembersDr. Lydia TapiaPostdocsDr. Bruna JacobsonGraduate StudentsKasra ManaviJon David CollaboratorsDr. Susan AtlasRelated ProjectsAntibody AggregationProtein Binding Site Flexibility |
Kinesin is a motor protein responsible for many cellular processes, including cargo transport and cell division. Its mechanochemical cycle consists of hydrolysis of one ATP molecule in each of the motor protein 8-nm steps towards the microtubule plus end. The structure of kinesin-1, an axonal cargo protein, comprises two catalytic domains (heads) that bind to the microtubule in alternating steps, a neck linker that joins the two heads to the stalk, and the cargo binding region at the end of the stalk. 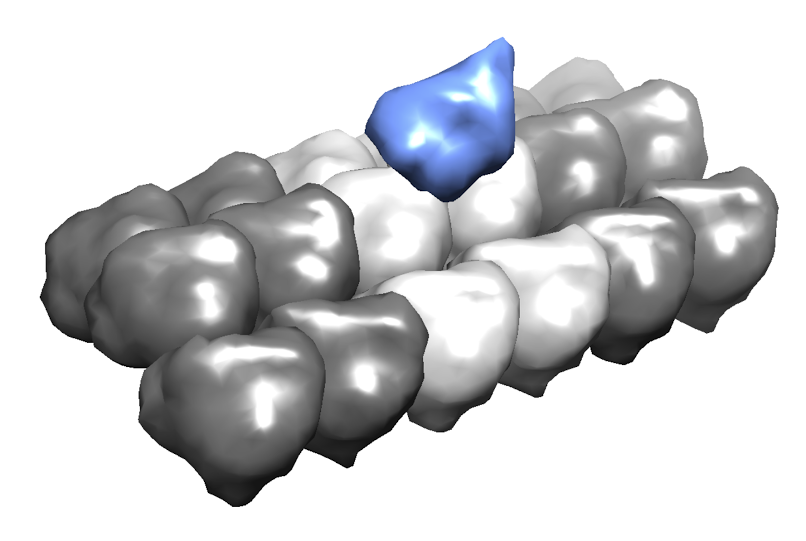 One kinesin head bound to the microtubule. Because the microtubule surface is most of the time crowded with other proteins in vivo, it is assumed that in order for a kinesin to shuttle cargo along a reasonable distance on the cell, it may eventually need to take side steps to neighboring protofilaments on the microtubule. This phenomenon has been observed experimentally but the energetics of the interactions between kinesin, tubulin dimers and molecules that work as obstacles is still not well understood. In an effort to characterize the kinesin motion on a crowded environment, we tackle this problem by making an analogy with robots avoiding collisions with obstacles in a 3D environment. By combining a 3D geometric model of the kinesin-microtubule system with energetics calculations, we can locate the configurations of the kinesin head that yield a low energy near the binding site, as seen in the figure. 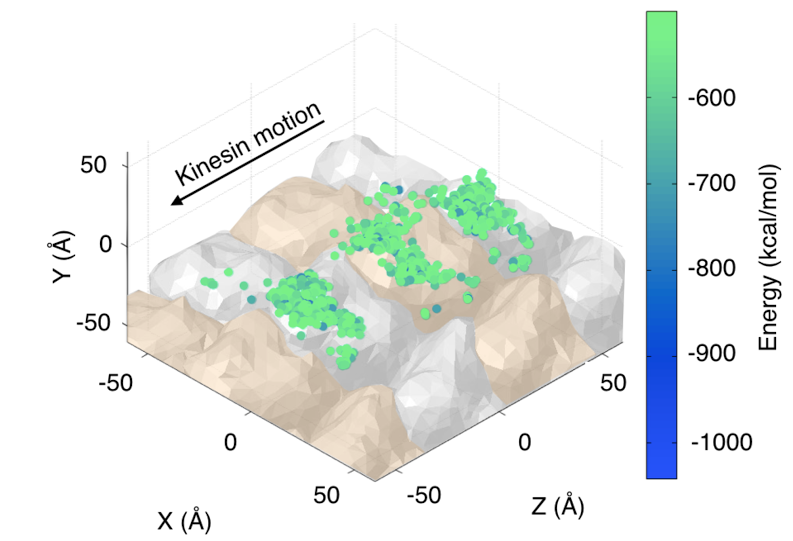 Low energy configurations of the kinesin-microtubule system. Publications & Papers |
