About the Project:
We are developing a dosimeter that is capable to directly measure linear energy transfer (LET) to allow physicians, medical physicists, and radiobiologists to study biological effects of different types of ionizing radiation used in external beam cancer therapy. This page will give you an up to date information of our development activities. Please scroll to the end of the page to sign up for email updates of our project.
Ionizing Radiation, Physical Dose, Biological Dose, RBE and LET
When radiation impinges on a living organism, it may transfer part or all of its energy to the medium. If this energy is sufficient, it may eject one or more orbital electrons and ionize the atoms or molecules in the medium, hence the name ionizing radiation. The energy dissipated per an individual ionizing event is about 33eV, more than enough to break a strong chemical bond such as C=C, which is only about 4.9eV, thus inducing massive biological damages to bio-molecules such as DNA, resulting in loss or alteration of the genetic information, and eventually cell death.
Physical dose is defined as the energy absorbed per unit mass and has a unit gray (1 gray = 1 Joule/kg). Most modern radiation therapy is planned by optimizing physical dose. The main objective is to create a dose distribution where the high dose region conforms to the tumor volume. Physical dose is employed as a principal measure of the quality of a treatment plan because it can be easily quantified via Monte Carlo simulations and experimental measurements using ion chambers or similar instruments.
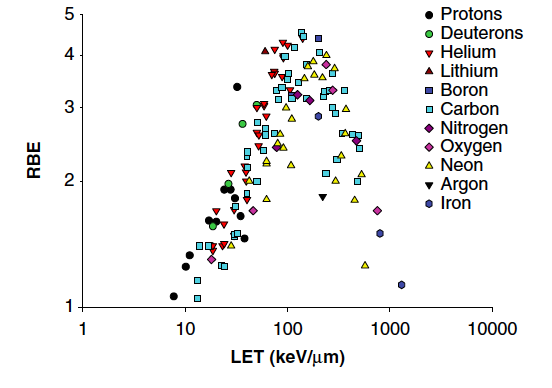
Using physical dose also has its limitations. Identical physical doses of different types of radiation may have a different biological effect. The cell killing ability, often referred to as the radiobiological effectiveness (RBE) is defined as the ratio of doses of a standard radiation D0 (e.g., from 60Co) and the test radiation Dr required to produce an equal biological effect: RBE = D0/Dr. Thus the higher the RBE, the less the dose to achieve the same biological effect, and the more effective the radiation in terms of cell killing. To put this into perspective, the RBE for carbon ions is, depending on tumor type and desired endpoint (biological effect), about 5 times that of X-rays. Compared to photon beam irradiation, the radiobiological effect (RBE) of hadron beams leads to a much higher level of complexity in treatment planning and application. Not only does it depend on the physical dose, but the RBE also varies along the beam path. RBE is a highly complex quantity depending on many physical and biological parameters, and therefore must be considered unsuitable for treatment planning of complicated cancer incidents. The physical quantity that can be used to characterize biological damage is the LET, the mean locally imparted energy to the medium by a particle. A general relationship between RBE and LET has been demonstrated in vitro and in-vivo in many cell lines and by many research groups. The Figure below is taken from a recent survey [Acta Oncologica, 2011(50), pp757] and shows a linear increase of RBE with LET up to a broad maximum where the effective RBE saturates and drops due to the overkill effect.

LET of ionizing particles in medium is the quotient dE/dl, where dE is the mean locally imparted energy to the medium by a particle traversing a distance of dl along the particle track. Note that LET is related, but not identical, to stopping power as it only considers the energy deposited within the close vicinity of the collision. In this context stopping power is sometimes referred to as unrestricted LET. Unlike in photon irradiation, where the ionization density (also referred to as the linear energy transfer (LET)) tends to be uniform throughout the irradiated area, the ionization density of hadron therapy also varies along the beam. Thus the variation of RBE with penetration depth into the human body potentially can be mimicked by the central physical quantity in ion-target interaction closely related to RBE, namely the LET. Precisely,
Ionization Chambers and Recombination Effects
To understand the design of our new LET dosimeter, we shall briefly describe the ionization chamber, probably the most important radiation dosimetry device. It usually consists of a gas or liquid filled enclosure between two electrodes (an anode and a cathode). A voltage is applied between the two electrodes. When the medium between the electrodes is ionized by the impinging radiation, the resultant positive and negative charge carriers move to their corresponding electrodes, thus creating a current that can be measured by an electrometer. The amount of charge collected can then be converted to the deposited dose.
Due to processes of diffusion, charge transfer, electron capture, or recombination the number of charge carriers collected at the electrodes is not identical to the number of charges generated in the active medium by the incoming radiation, a signal loss effect commonly described as quenching. To obtain an accurate reading of the dose delivered, corrections for these losses of charges have to be applied.
Recombination effects in ion chambers can be classified in two main categories: initial recombination and general recombination. Initial recombination occurs during the first tens of nanoseconds along the track of one individual primary particle. Theoretical description of initial recombination further distinguishes between geminate recombination, involving one electron and its mother ion, and columnar recombination, in which clusters formed along a single track begin to expand due to ionic and electronic diffusion and start to overlap. Initial recombination depends on the properties of the medium, its temperature, and on the external electric field E, but not on the dose rate. Obviously it also depends on the density of ionizing events along the primary particle track, and therefore on the linear energy transfer (LET) of the primary beam.
General recombination (or volume recombination) occurs between charges produced by independent tracks produced by different primary particles. Charge carriers that escaped initial recombination can diffuse through the medium and electrons and ions originating from different primary particles can interact and recombine. General recombination therefore depends, in addition to the liquid properties and the applied field, on the dose rate, but not on LET.
Our LET Dosimeter
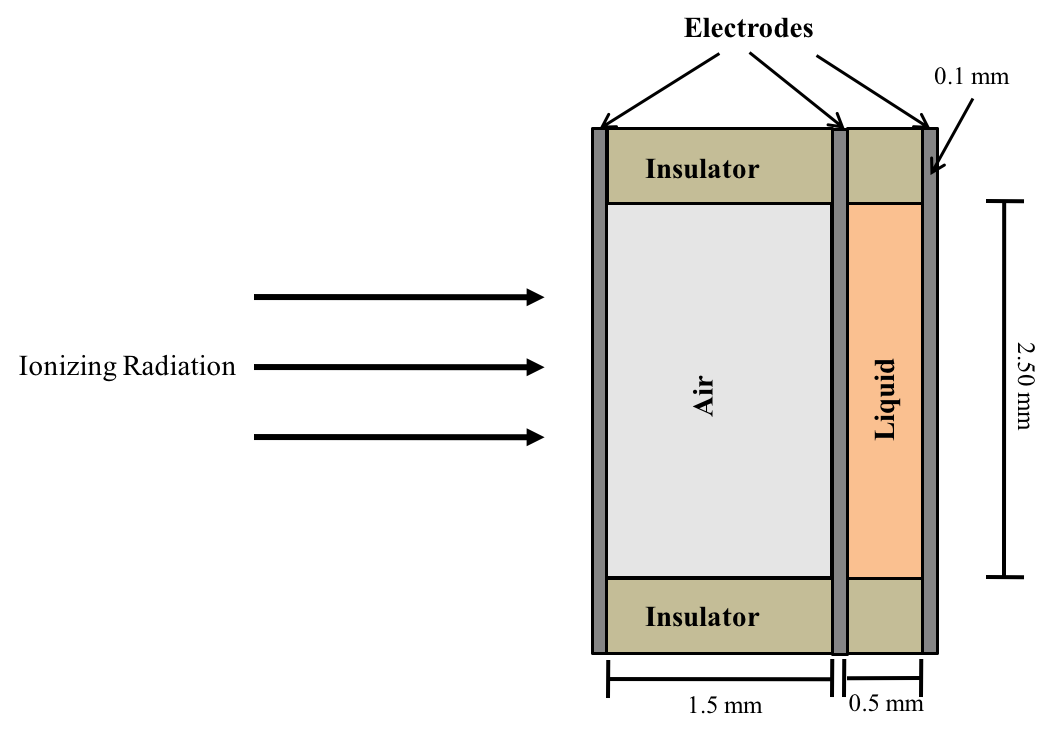
Our new LET dosimeter is based on the different quenching effects of air-filled ionization chambers and liquid-filled ionization chambers.
The air-filled ionization chamber is mostly commonly used for measuring radiaiton dose. Generally speaking, in an air-filled ionization chamber, both initial and general recombination effects can be ignored.
In recent years, liquid-filled ionization chambers have also been investigated as an alternative device for measuring radiation dose. The key advantage of a liquid-filled ionization chamber (LIC) over the standard air filled chamber (IC) results from the significantly higher density of the liquid, allowing the active volume to be much smaller and offering a higher spatial resolution. In addition to higher spatial resolution, LICs are water equivalent, show long-term stability, and have small directional dependency. However, all these advantages of LICs come with at least one significant drawback. Due to the higher density of the liquid, the distances between ionization events are much shorter than in an air-filled IC. This higher density of charge carriers together with the low ion mobility in the liquid increases the probability of electron-ion recombination before the charges can be collected by the electrodes, resulting in a significant signal quenching.
Our idea for measuring LET is that if one can somehow find out the relative signal quenching in a LIC due to the LET dependent initial recombination, then one can extract the LET of the beam. To achieve this, one will need to (1) suppress the signal quenching due to general combination, and (2) find out the total amount of charges without quenching. To achieve (1), we note that general recombination in the LIC can be suppressed by strong electric field. To achieve (2), we note that due to the much lower density of the medium in air-filled ionization chambers (ICs), both initial and general recombination can be considered negligible in most applications. This implies that if one places an IC and a LIC at the same position, the IC reading can be viewed as the "normalized" amount of the total charges released before initial and general recombination in the LIC.
Specifically, we propose to measure LET by placing an IC and a LIC at the same location in a medium (e.g. water). Let QIC and QLIC be their respective charges collected. Assuming that initial and general recombination in the IC can be ignored, and the general recombination in the LIC is suppressed by the applied electric field, then the ratio QIC/QLIC, which we define as the recombination index, is obviously dependent on the LET.
The Figure below shows the schematic design of our LET dosimeter. Several prototypes are currently being built and tested. Stay tuned for updates.
Research Update:
Filling in a liquid ionization chamber ...
Research Update:
We have finished the design of the version 3 of our ion chamebr electrodes. Several chambers have been assembled and have been irridiated under 6MV X-ray beams at the UNMCCC.
Here is a working chamber.
Here is a working chamber in its assembly for future irridation under hadron beams.
Research Update:
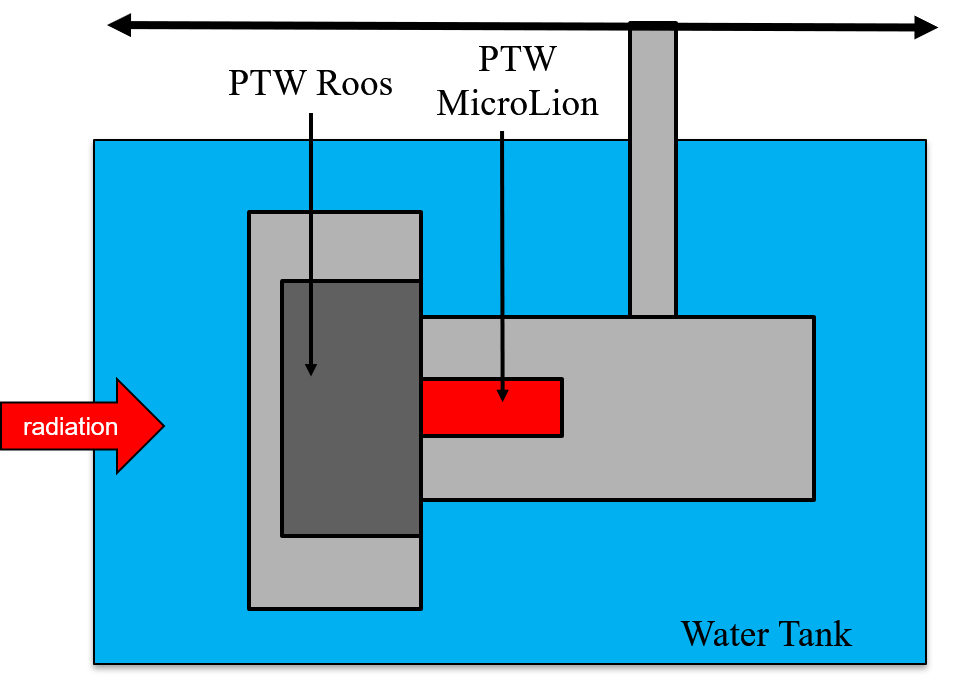
A dual chamber assembly consisting of a PTW Roos Type 34073 (IC) and a PTW MicroLion (LIC) was constructed. The assembly was irradiated under proton, carbon and oxygen ion beams at two different energies at different depths along the beam direction at Heidelberg Ion Therapy Center.
Research Update:
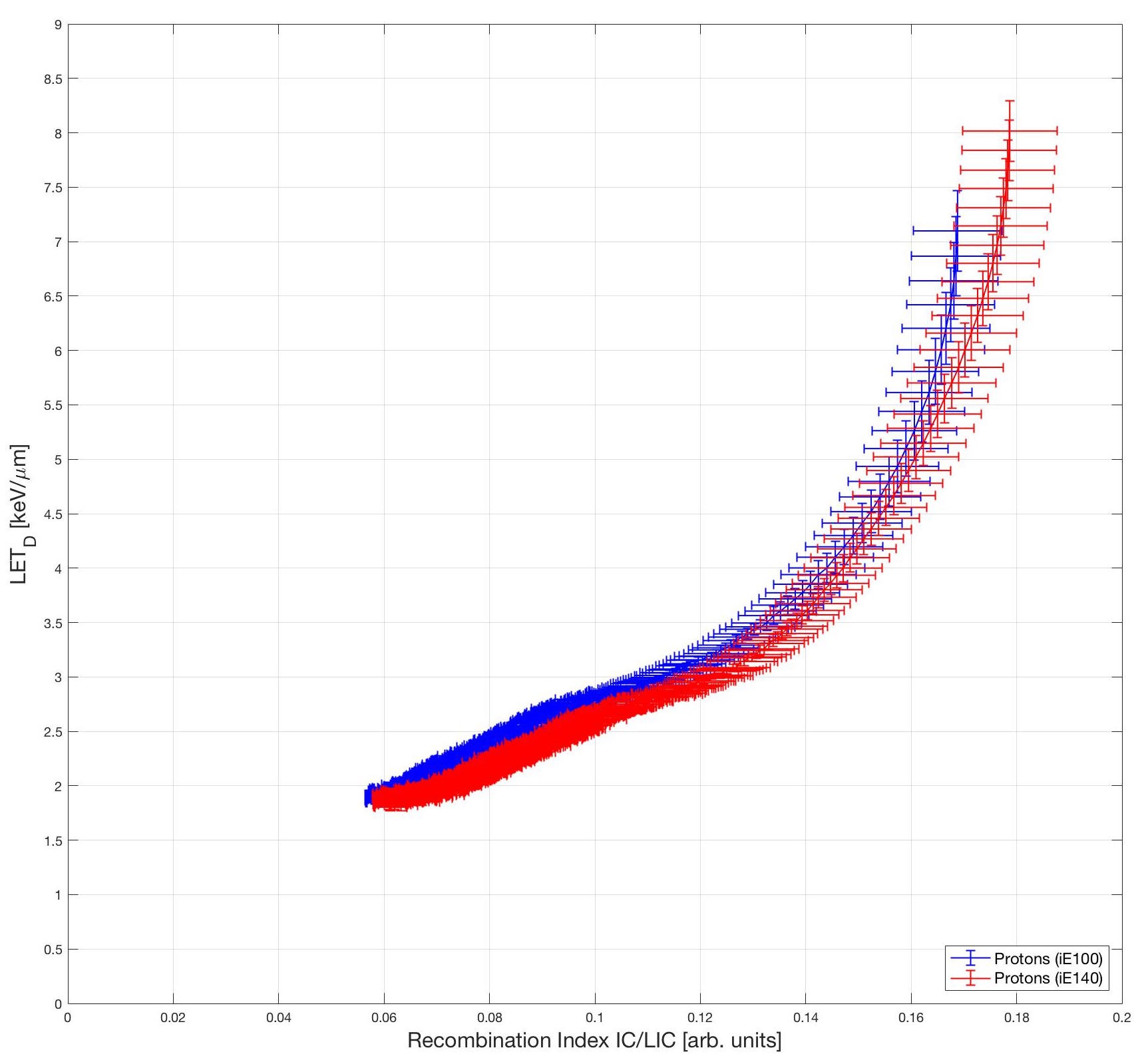
The ratio between the two chamber readings were curve fitted to the LET values obtained by Monte Carlo calculations to produce the calibration curves. Monte Carlo simulations were calculated on two Monte Carlo codes and are cross-compared to ensure accuracy. The two Monte Carlo codes used are FLUKA (http://www.fluka.org/fluka.php) and SHIELD-HIT (https://shieldhit.org/). Here are the calibration curves for protons. The red and blue data are for two different energies. Obverse the energy independence of the curves.
Here are the calibration curves for protons. The red and blue data are for two different energies. Obverse the energy independence of the curves.
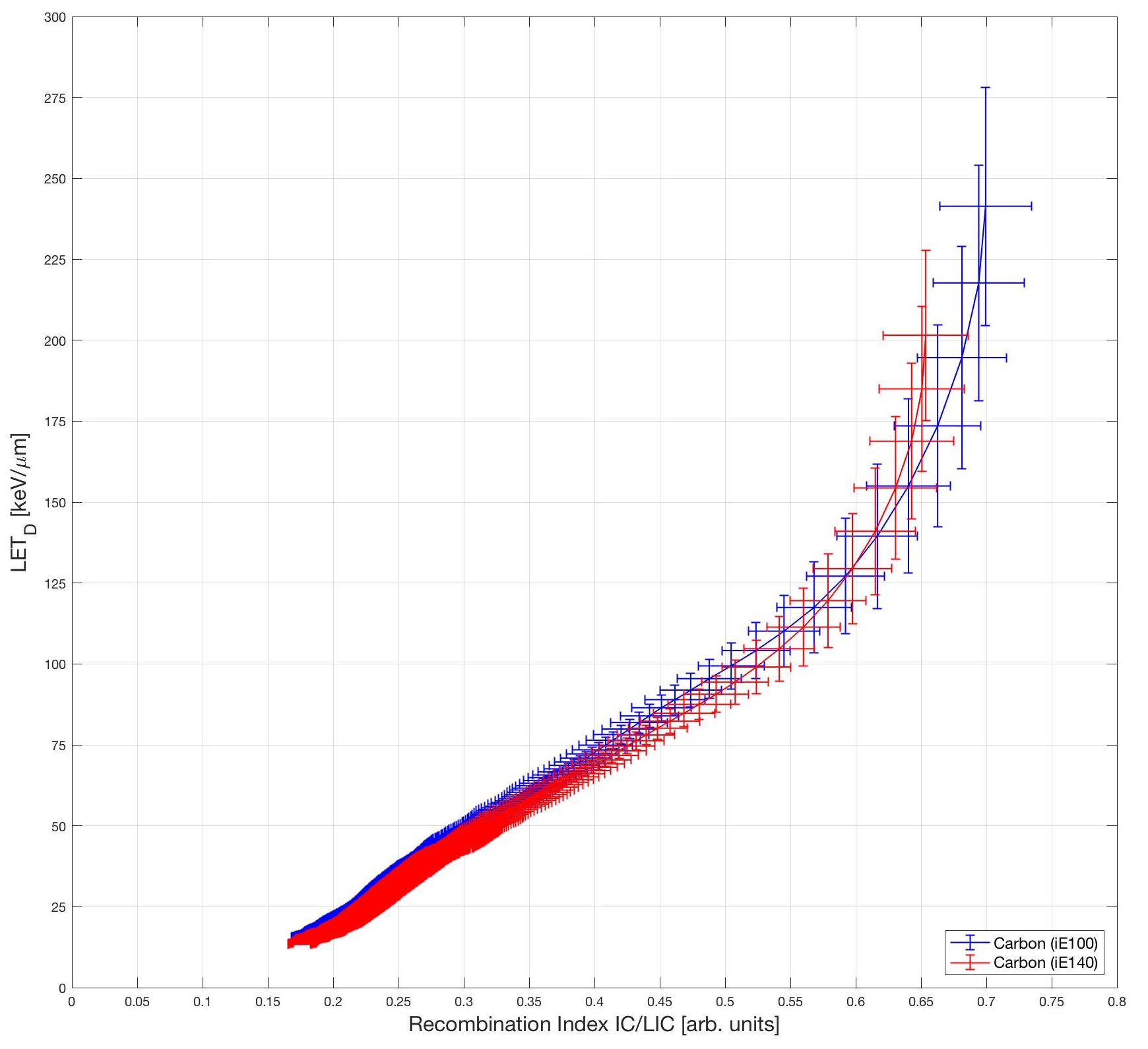
Here are the calibration curves for carbon ions. The red and blue data are for two different energies. Obverse the energy independence of the curves.
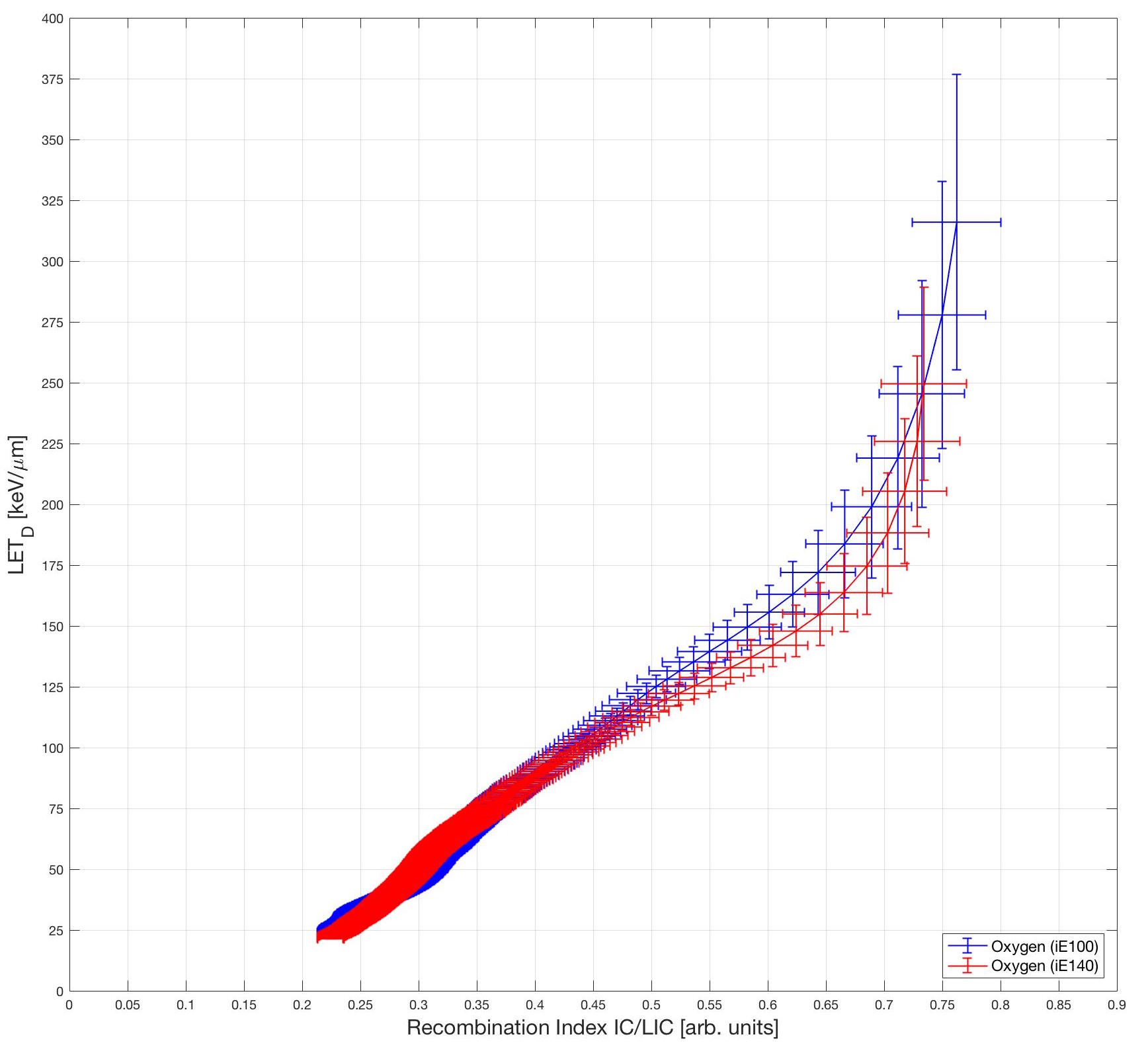
Here are the calibration curves for Oxygen ions. The red and blue data are for two different energies. Obverse the energy independence of the curves.
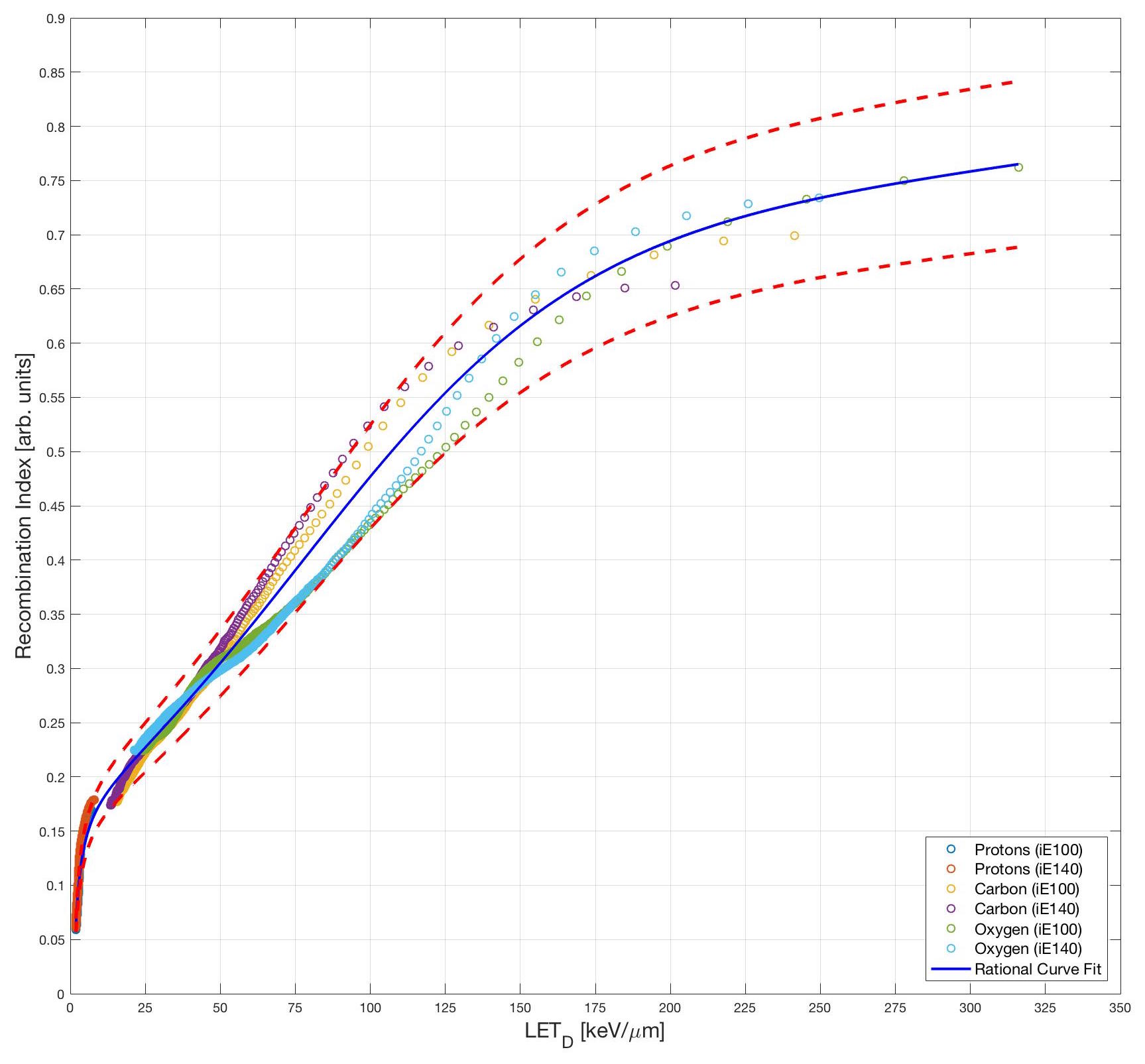
However, the most surprising aspect of the data is that when we combine all the ion species, the calibration curves indicates that the technique is also ion species independent and can produce LET measurements within an error band of +/- 10%.
Research Update:
Here is the data from our summer experiment at Heidelberg Ion Therapy Center: Please refer to Stephen's thesis for how the details of data processing.
Research Update:
We were very surprised that the proposed technique is not only energy independent, but also ion species independent. The calibration curve for LET can be obtained with either polynomial fitting or Gaussian fitting. However, it is not clear if these curve fitting results reveal the true mathematical relation between the LET and recombination index. At this point, we are doing some theoretical research to see if we can find any meaningful mathematical and physical explanation of the relation. We will keep you posted if we have made any progress.
Our Team:
Shuang (Sean) Luan, Professor of Computer Science, University of New Mexico
Michael Holzscheiter, Research Professor of Physics, University of New Mexico
Stephen Bello, PhD. Stephen did his PhD research developing the LET dosimeter technology. He graduated with a PhD in Physics from University of New Mexico in December 2017. Here is Stephen's dissertation.
Greg Iven, MS Student of Computer Science, University of New Mexico
Recent Publications:
Tegami S, Bello SD, Luan S, Mairani A, Parodi K, Holzscheiter M. LET Monitoring using Liquid Ionization Chambers. International Journal of Medical Physics, Clinical Engineering and Radiation Oncology. 2017 6(2).
Bello S, Luan S, Holzscheiter M. A method for measuring LET of hadron beams. The 60th Annual Meeting of American Association of Physicists in Medicine (AAPM), 2018. Oral Presentation. (Our talk is on July 29th in Karl Dean Ballroom A2. See you in Nashville in July 2018!)
Email List:
Thank you for visiting our page on LET dosimeter. To get updates of our project, please email sluan@unm.edu with the subject: LET Dosimeter, and include your contact information (name, title and affliation) in the body of the message.
Acknowledgement:
NIH R21 CA197325-02 An LET Dosimeter.