Exploring Major Histocompatibility Complex Binding Site Flexibility
Faculty MembersDr. Lydia TapiaGraduate StudentsTorin AdamsonAlumniJohn BaxterLidong Wang Related ProjectsAntibody AggregationKinesin Procession |
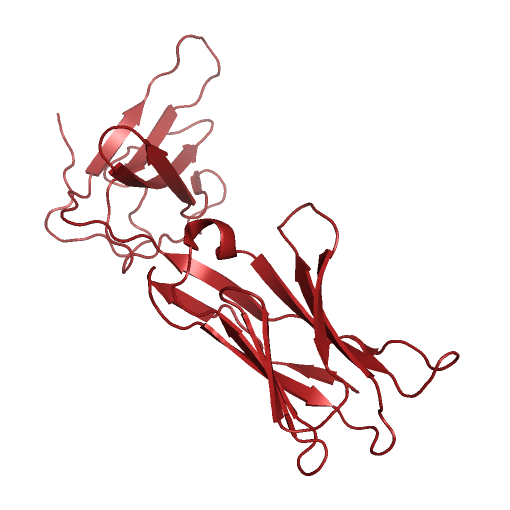
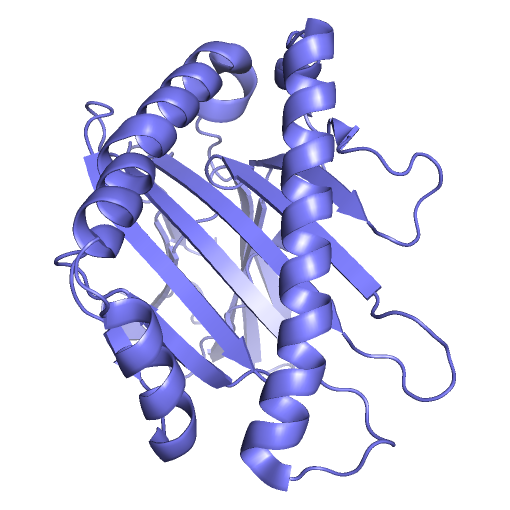
Project BackgroundT-Cell Receptors (TCR), found on the surface of T-Cell white blood cells, initiate adaptive immune responses when they react with antigens under the right conditions. The body produces T-Cells with many different TCR variations, allowing the immune system to recognize a large variety of pathogens. T-Cells that bind with antigen under the right conditions reproduce quickly, producing a large number of white blood cells recognizing that antigen. White blood cells with antigen binding TCR act as memory that something shaped like the original antigen was deemed a threat during an earlier immune response. In order for an antigen to bind with TCR and be deemed a threat by the adaptive immune system, several conditions must be met. One of these conditions is that the antigen must bind to, and be presented by, a Major Histocompatibility Complex (MHC) molecule. MHC molecules involved in the presentation of antibodies to TCR have a binding groove with a beta-sheet floor and flexible alpha-helix walls. As antigens must bind within MHC's binding groove in order to be presentable to TCR, knowing what kinds of molecules could or could not potentially enter MHC's binding groove is important to the development of new vaccines. Computer simulations of such protein systems can provide insight into the binding affinities between MHC and ligands of interest. In the past, such systems have typically been modeled using fixed geometric representations of the binding site cavities. However, such models are not able to take into account the flexibility of receptor structure, and are therefore limited in their accuracy to predict the feasibility of binding ligands that require receptor flexibility. Highly accurate physics-based molecular dynamics simulations are capable of modeling protein structure flexibility, but these approaches are typically too computationally demanding for long time scale simulations. 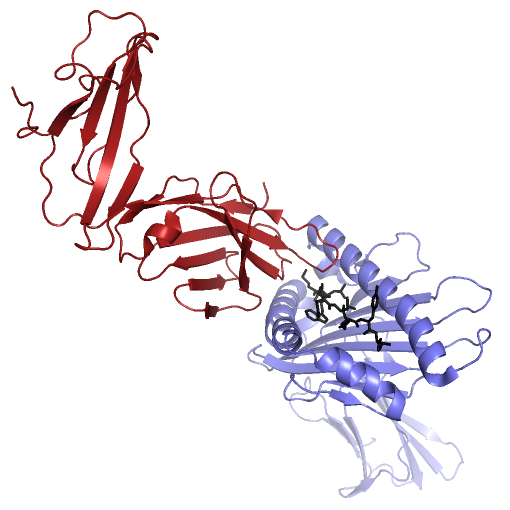 MHC (PDB ID: 3H9S, Chain A, in blue) presenting the TEL1P peptide (PDB ID: 3H9S, Chain C, in black) to TCR (PDB ID: 3H9S, Chain D, in red). Project DescriptionWe are investigating ways to advance another approach, based on a probabilistic roadmap framework, that has already proven capable of efficiently capturing biologically relevant features of molecular energy landscapes, but that will need to be further improved in order to provide the basis for accurate simulations of large molecular interactions like those that are found in the MHC - Ligand - TCR system. VideosMolecular Dynamics Simulation of MHC (PDB id: 3H9Sa) Alone, With Binding Site in Greenhttp://www.cs.unm.edu/tapialab/Research/FlexibleMHC/Movie/MD/MHC/moviewithoutwater.aviMolecular Dynamics Simulation of MHC (PDB id: 3IXAa) and TAX Peptide (PDB id: 3IXAc); Pepdide Shown in Bluehttp://www.cs.unm.edu/tapialab/Research/FlexibleMHC/Movie/MD/TAX/TAXNW.aviMolecular Dynamics simulation of MHC (PDB id: 3H9Sa) and TEL1P Peptide (PDB id: 3H9Sc); Peptide Shown in Bluehttp://www.cs.unm.edu/tapialab/Research/FlexibleMHC/Movie/MD/TEL1P/TEL1pNw.avi |